VYXEOS HAS A DEMONSTRATED SAFETY PROFILE
OVERALL FREQUENCY AND SEVERITY OF ADVERSE EVENTS WAS COMPARABLE FOR VYXEOS AND CONVENTIONAL CHEMOTHERAPY1*†
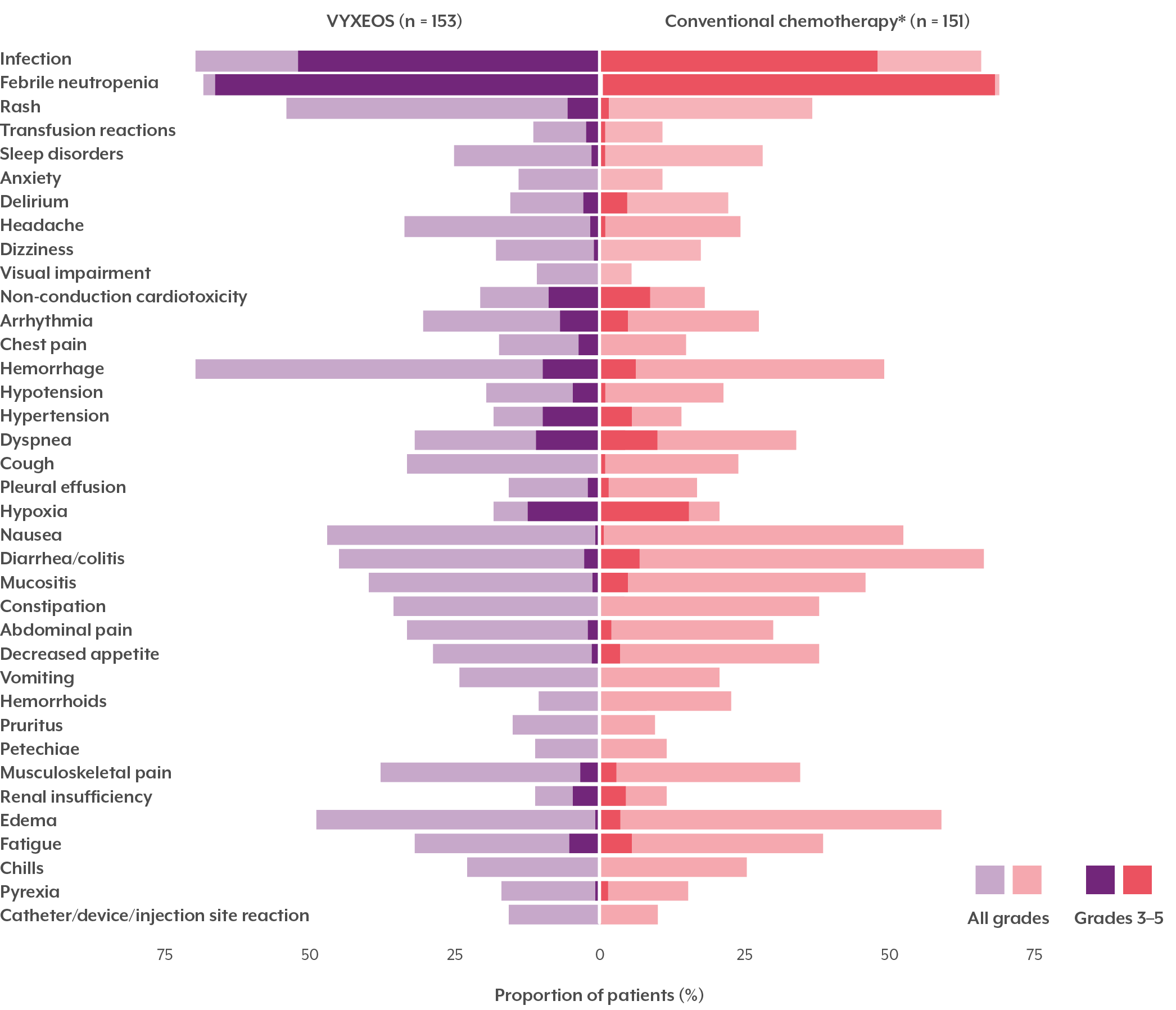
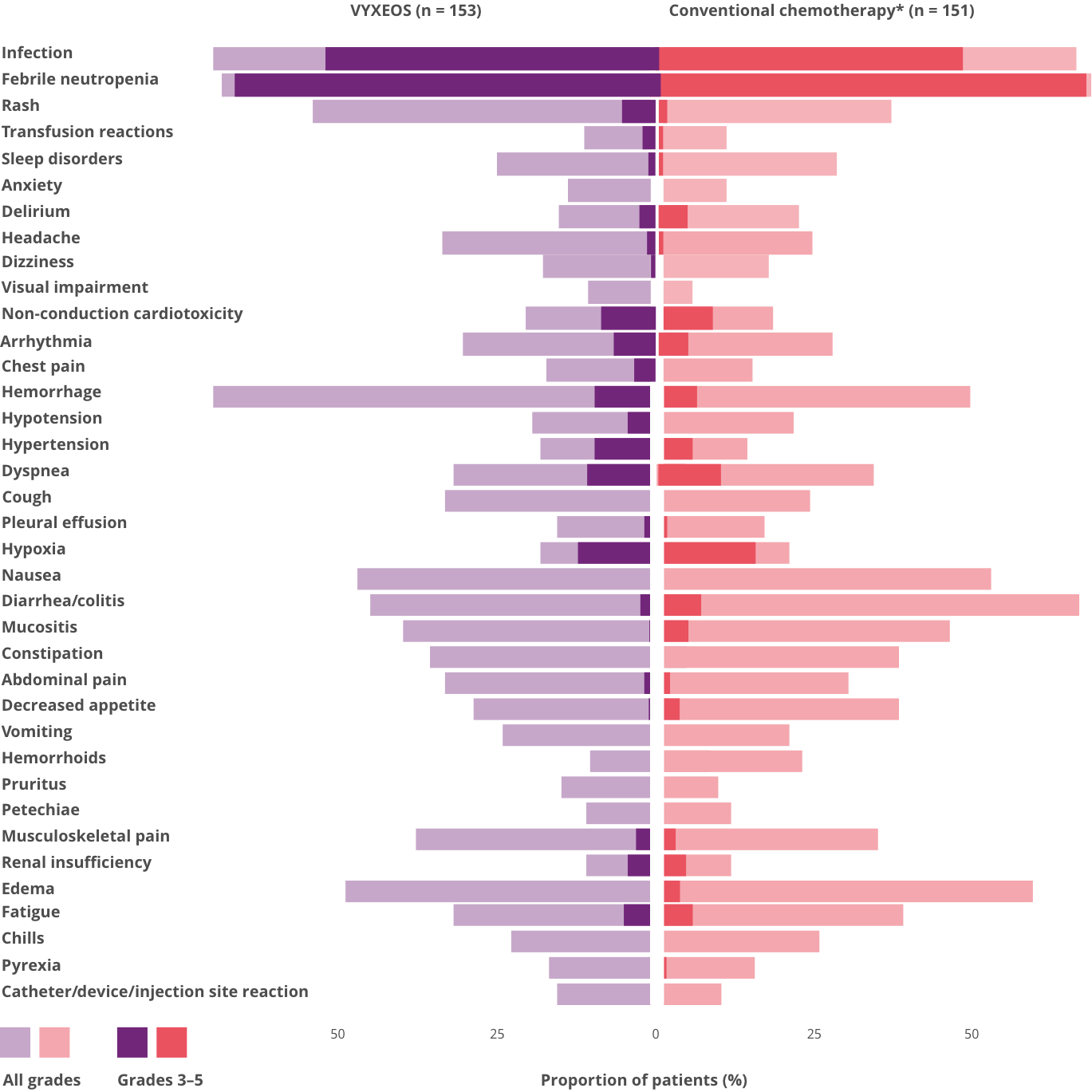
COMMON ADVERSE REACTIONS (≥ 10% INCIDENCE IN THE VYXEOS ARM) DURING THE INDUCTION PHASE‡
18 % (28/153) of patients treated with VYXEOS discontinued treatment due to adverse events
vs
13 % (20/151) of patients treated with conventional chemotherapy*
PATIENTS MAY REQUIRE ADDITIONAL MONITORING AS VYXEOS IS ASSOCIATED WITH PROLONGED THROMBOCYTOPENIA AND NEUTROPENIA VS CONVENTIONAL CHEMOTHERAPY1,2*
| VYXEOS | CONVENTIONAL CHEMOTHERAPY* | |
|---|---|---|
| Median time to recovery from thrombocytopenia (≥ 50,000/μl) after first induction for patients who had achieved CR/CRi1,2 |  |  |
| Median time to recovery from neutropenia (ANC value ≥ 500/µl) after first induction for patients who had achieved CR/CRi1 |  |  |
Adapted from the VYXEOS Product Monograph and Lancet, et al (2018).