VYXEOS CAN BE ADMINISTERED IN 90-MINUTE INFUSIONS FOR BOTH INDUCTION AND CONSOLIDATION IN ADULTS WITH HIGH-RISK AML*
DOSING CONSIDERATIONS WITH VYXEOS
- Treatment should be initiated and monitored under the supervision of a physician experienced in the use of chemotherapeutic medicinal products
- VYXEOS must not be interchanged with other daunorubicin and/or cytarabine-containing products
- Patients may be pre-medicated for nausea and vomiting. An anti-hyperuricemic therapy should be considered (e.g., allopurinol) prior to initiating VYXEOS
Treatment should be continued as long as patients continue to benefit or until disease progression, up to a maximum of 2 courses for induction and consolidation each.


After the first induction, subsequent induction courses may be:
- Administered after 2–5 weeks in patients who do not achieve remission and show no unacceptable toxicity
- Required for patients who attain normal-appearing bone marrow
After the start of the first consolidation, subsequent courses may be:
- Administered within 5–8 weeks in patients who do not show disease progression or unacceptable toxicity
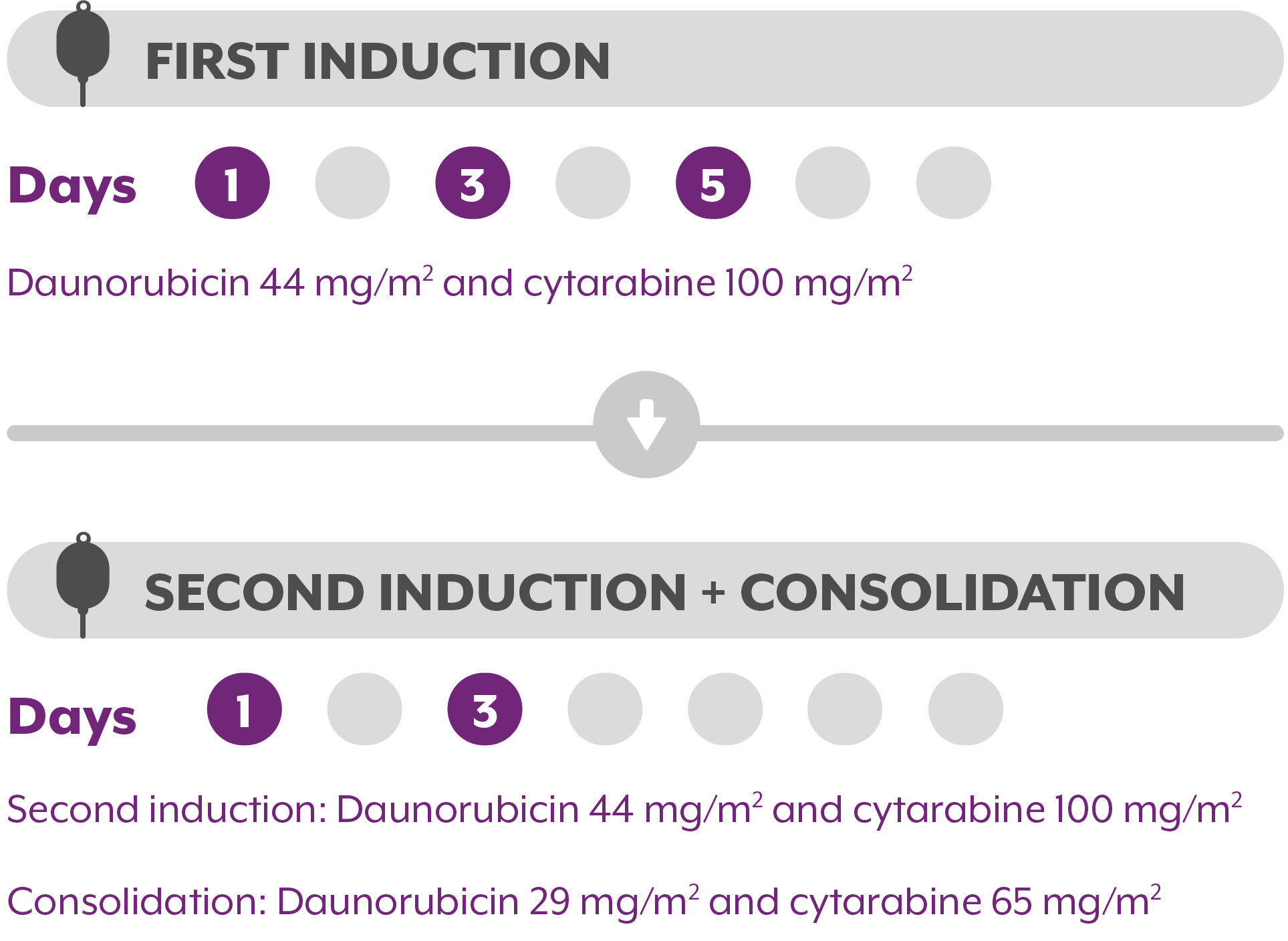
RECOMMENDED DOSING SCHEDULE WITH VYXEOS

Missed a dose?
If a planned dose of VYXEOS is missed, administer the dose as soon as possible and adjust the dosing schedule accordingly with treatment interval maintained.
VYXEOS preparation and administration
Learn more