WHO 2016: CLASSIFICATION OF HIGH-RISK AML SUBTYPES: t-AML AND AML-MRC
THERAPY-RELATED AML (T-AML)
According to WHO, t-AML is defined as previously untreated AML with a history of prior cytotoxic therapy1,2*
Cytotoxic therapies associated with development of t-AML2 :
- Alkylating agents and radiation
- Latency period of 4–10 years
- Topoisomerase II inhibitors
- Latency period of 2–3 years
- Immunosuppressive therapies
- Latency period of 3–4 years
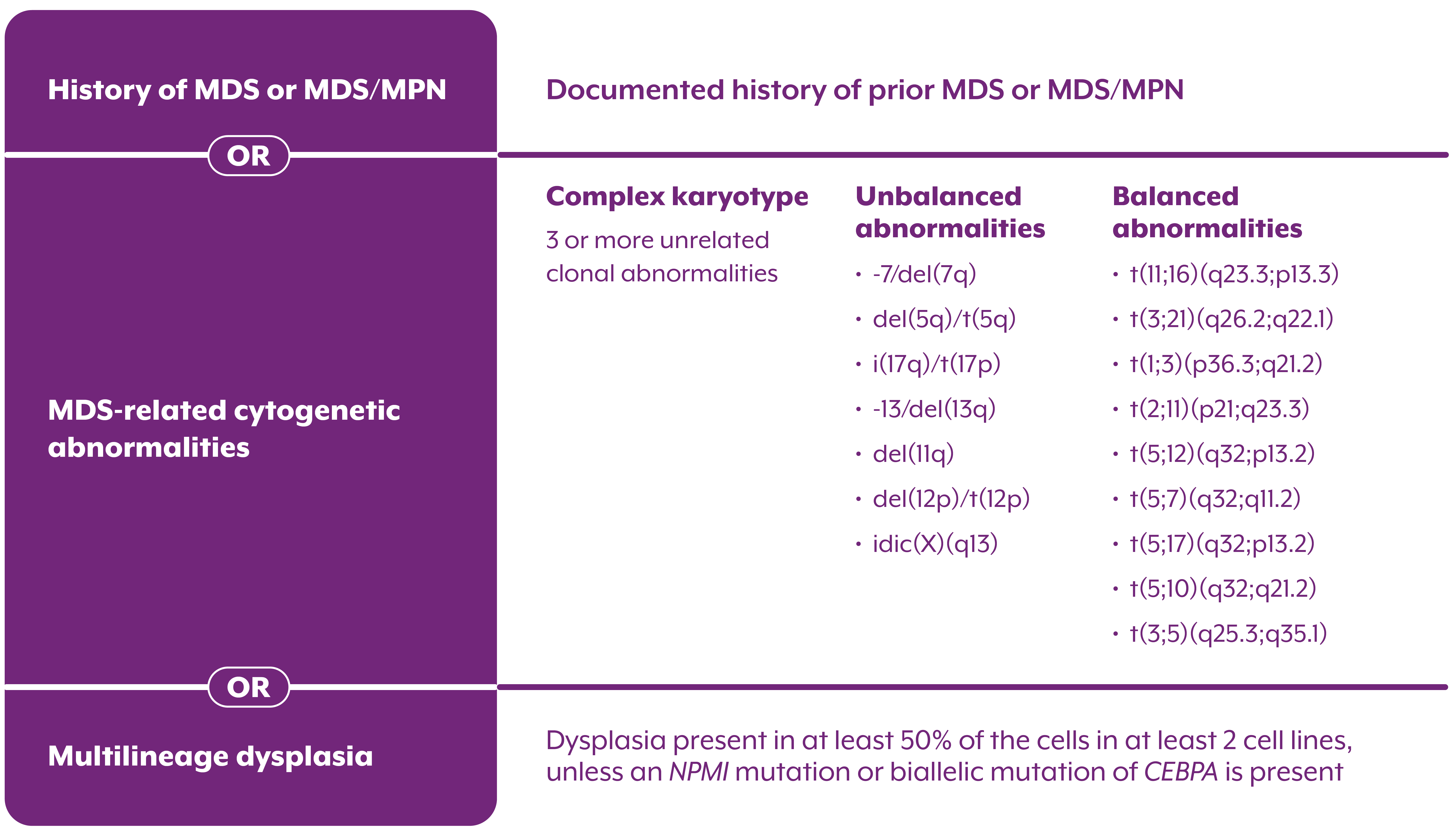
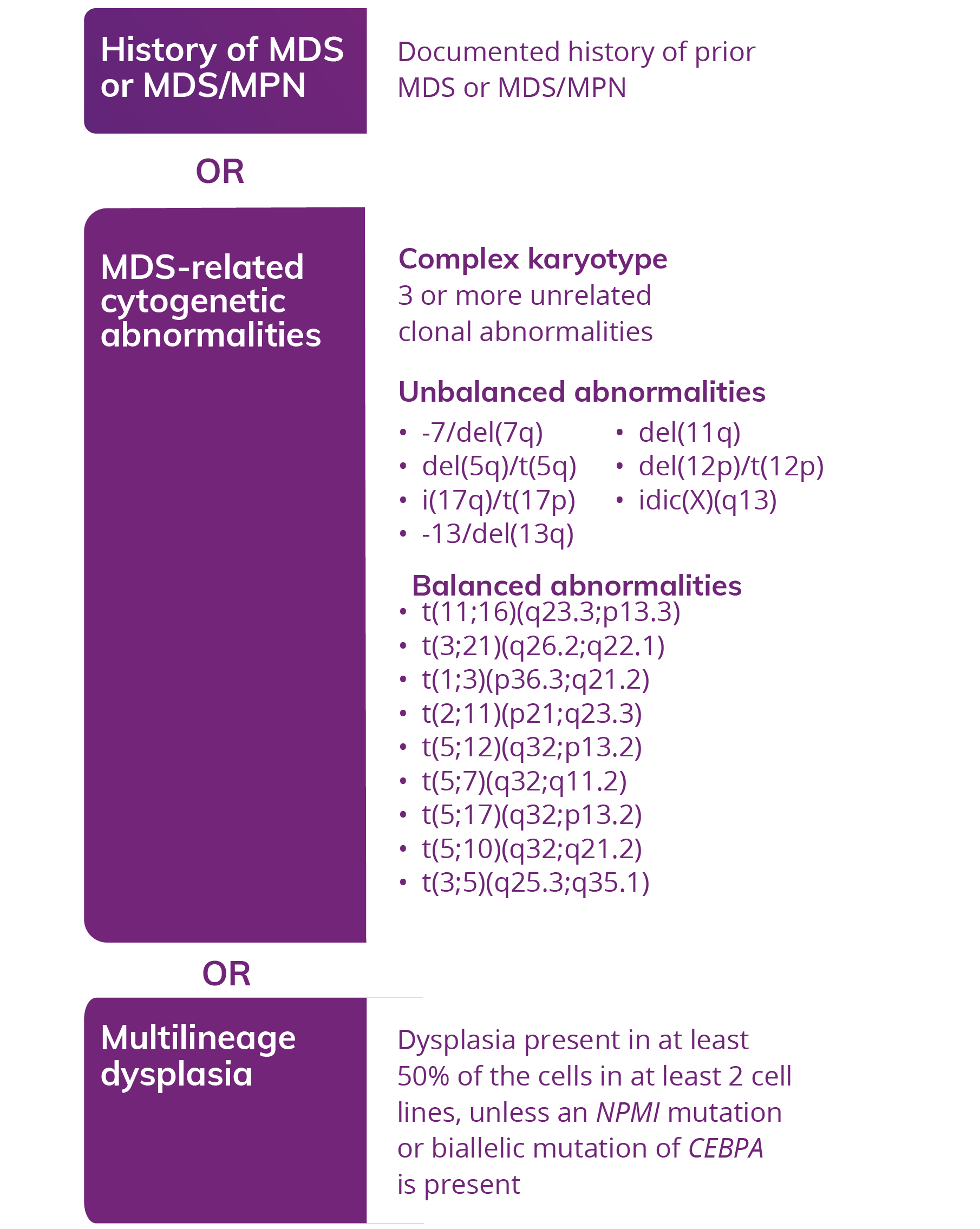
AML, WITH MYELODYSPLASIA-RELATED CHANGES (AML-MRC)
AML: ≥20% blasts in peripheral blood2
AML arising from a prior MDS or an MDS/MPN2
OR
AML with an MDS-related cytogenetic abnormality2
OR
AML with multilineage dysplasia2
A diagnosis of AML-MRC may require cytogenetic testing or a morphology exam for confirmation1
The classification of AML has been updated to reflect breakthroughs in disease understanding1,3,4
- The VYXEOS indication is based on the WHO 2016 classification of AML1
- The 2022 WHO and ICC classifications have revised the definition of AML-MRC and t-AML3,4
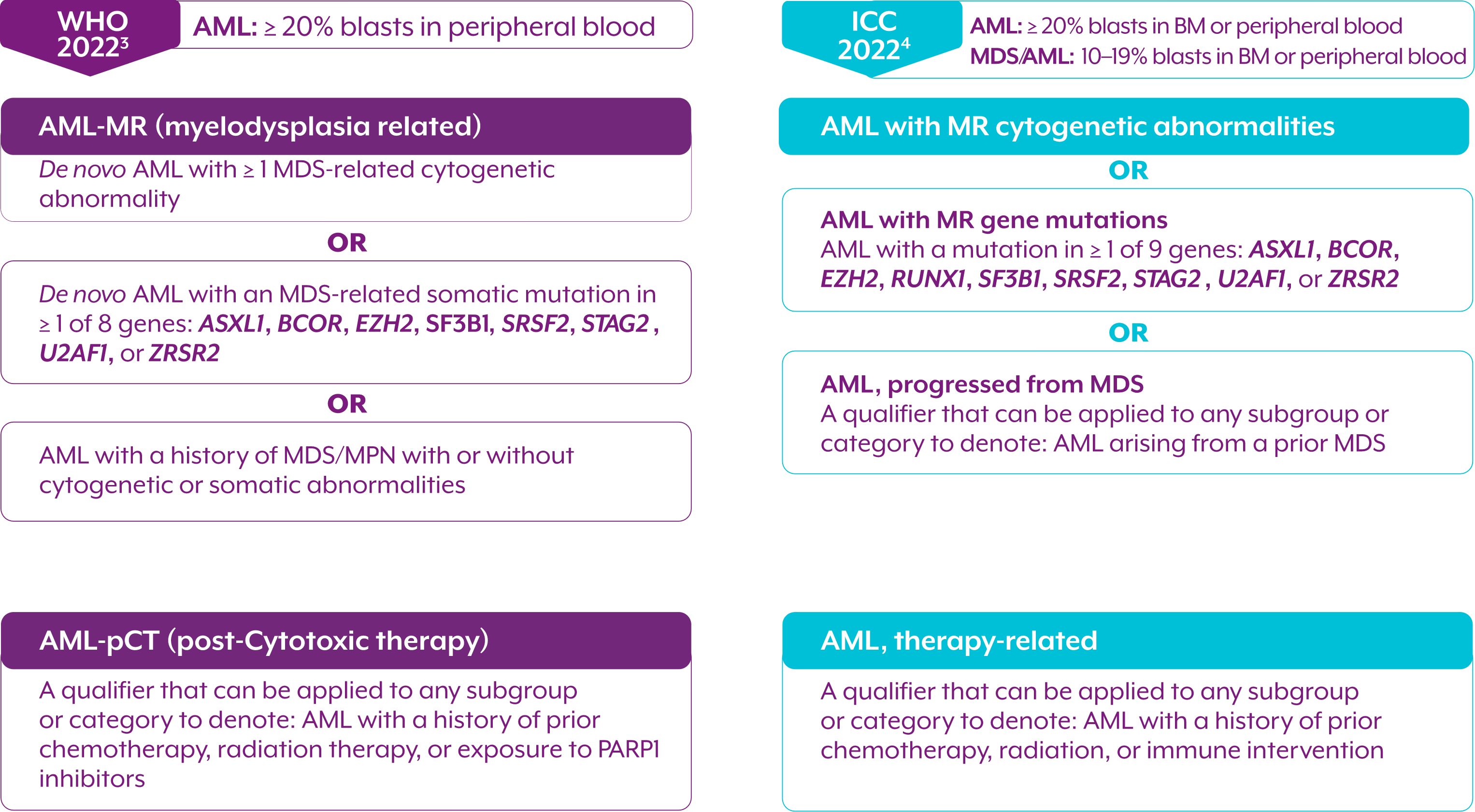
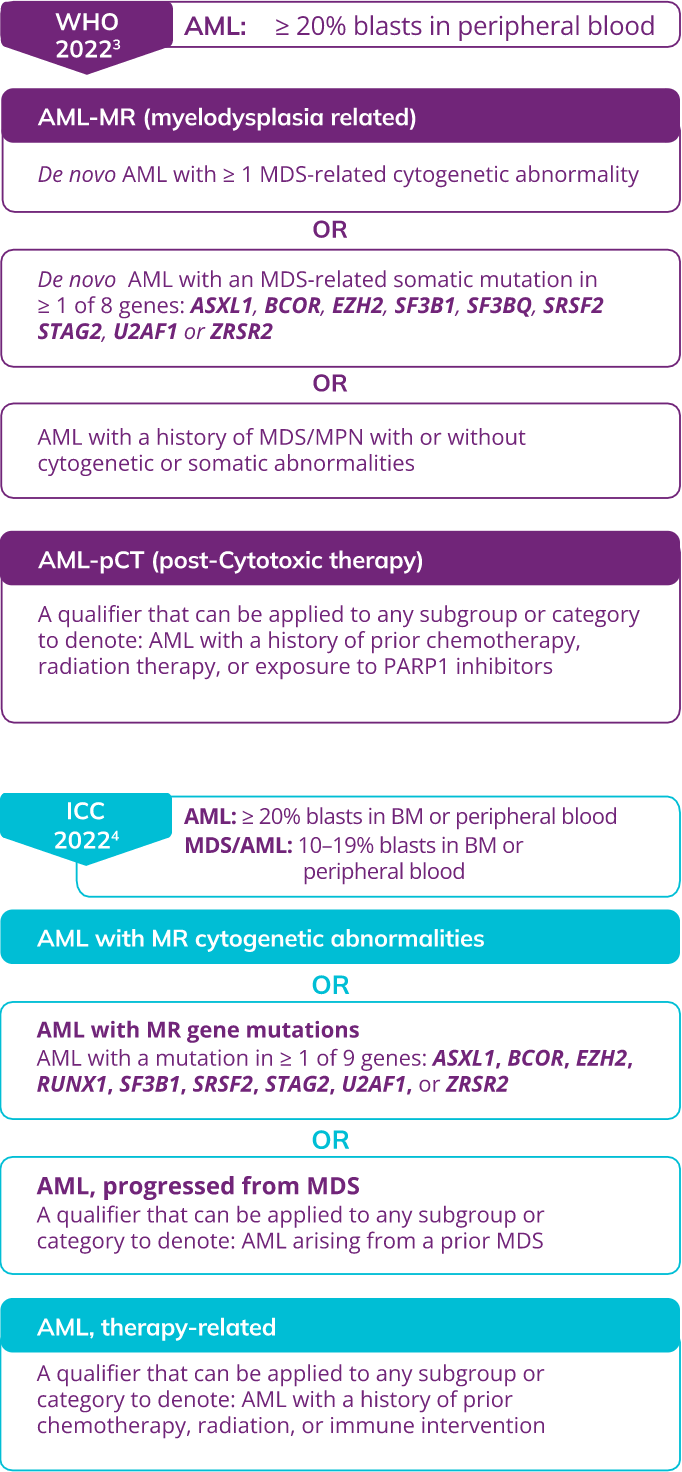
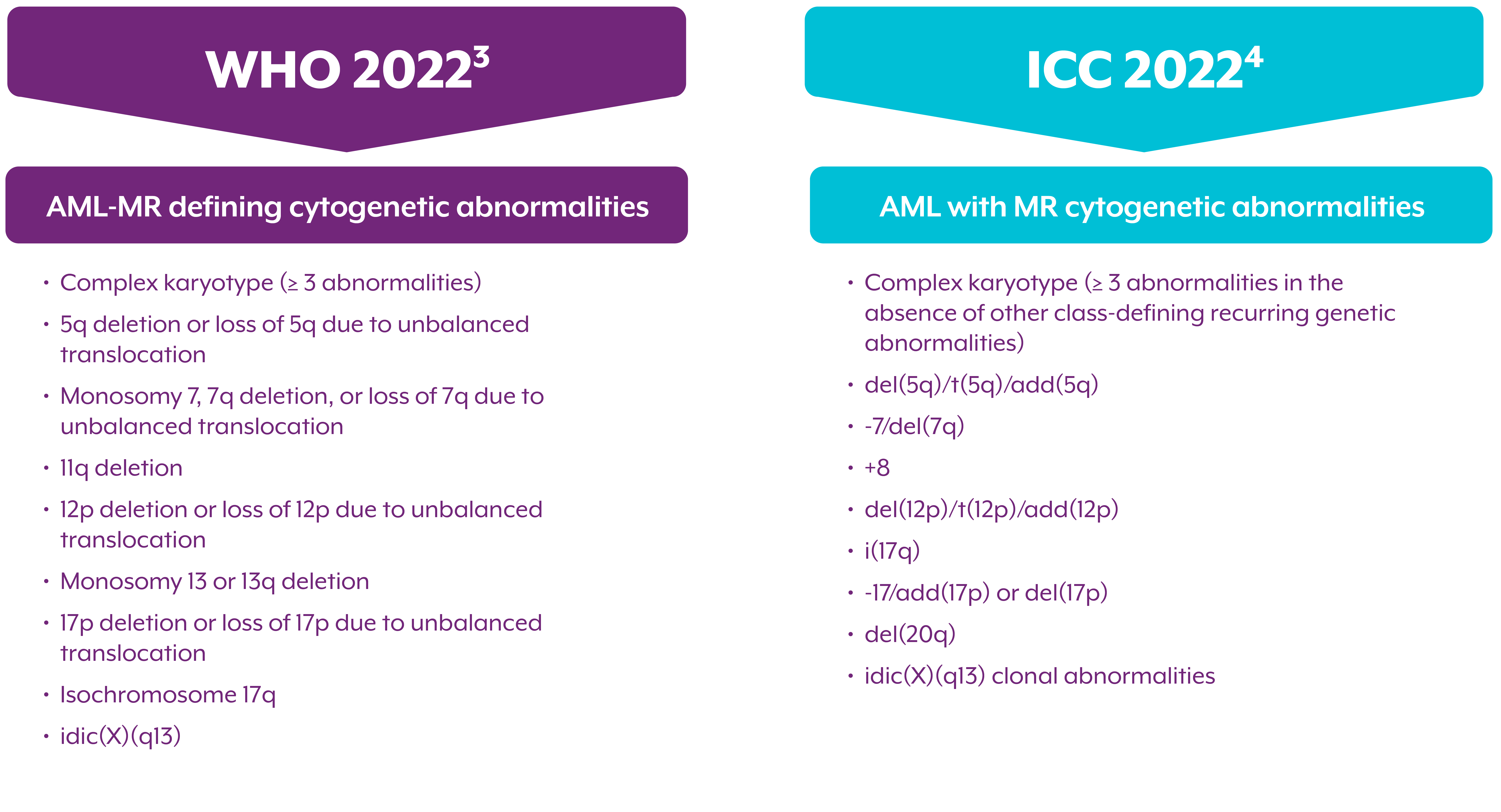
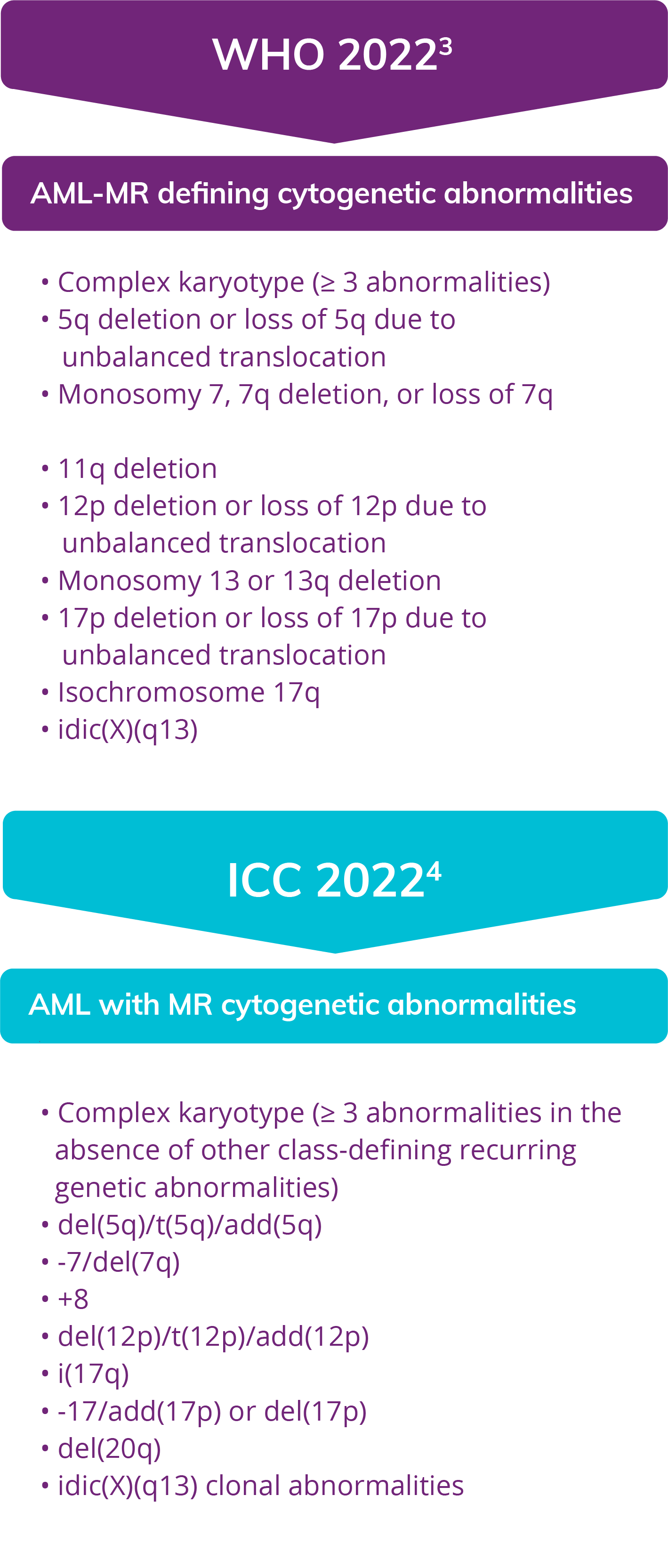
Myelodysplasia-related cytogenetic abnormalities as described in the WHO 2022 and ICC 2022 Guidelines
The WHO 2022 and ICC classifications share overlapping cytogenetic abnormalities